Russian Covid 19 Vaccine Sputnik V gets approval for clinical trials in India
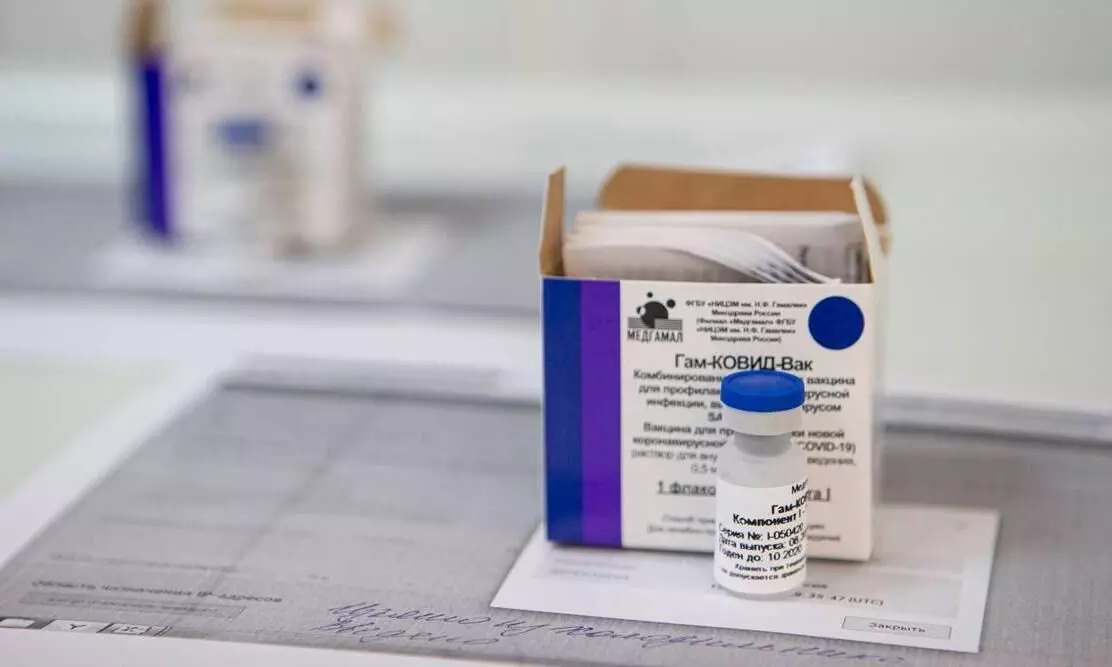
text_fieldsA view of a vial of Russia's experimental Sputnik V coronavirus vaccine. (Photo: AP)
New Delhi: Russian Covid 19 Vaccine gets nod for clinical trials in India. Dr. Reddy's group have announced that after striking down the first proposal, they have received approval for conducting clinical trials for the vaccine,Sputnik V in India from the Drug Controller General (DCGI).
"We acknowledge DCGI's scientific rigour and guidance in the entire process",said G V Prasad, Co-chairman and Managing Director of Dr Reddy's Laboratories.
"This is a significant development that allows us to commence the clinical trial in India and we are committed to bringing in a safe and efficacious vaccine to combat the pandemic," Prasad added
Russia is the first country to grant regulatory approval for Corona virus vaccine after small scale trials. A Phase III trials of Sputnik V engaging 40,000 participants is currently underway in Russia.
On September 16 , Dr Reddy's group entered into a partnership to conduct clinical trials and distribution of Sputnik V vaccine in India. As part of the partnership, 100 million doses of Sputnik will be supplied in India.
The initial proposal of testing in larger population like India was turned down in concern with Sputnik V's safety.
(Translated by Farha Zulthana)