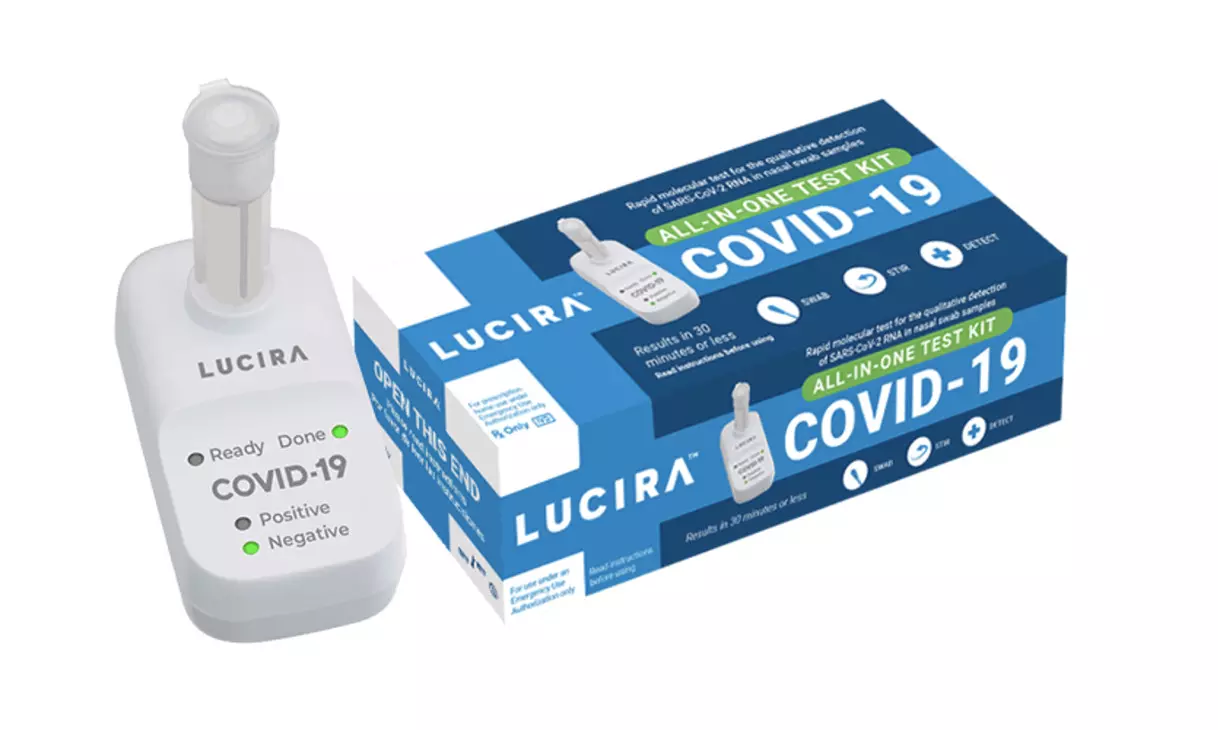
USFDA authorizes first at-home Covid-19 test kit
text_fieldsHong Kong/ New York: The U.S. approved the first COVID-19 All-In-One Test Kit for home use that provides results within 30 minutes. This is anticipated to contribute considerably in battling the pandemic as testing capabilities come under heavy strain nationwide.
The single-use test, made by Lucira Health, has been given emergency use authorization for home use with self-collected nasal swab samples in individuals of age 14 and above who are suspected of COVID-19 by their health care provider, the US Food and Drug Administration said on Tuesday. With a prescription, people can acquire it for around $50, as it said to be accessible across the country by spring 2021.
While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home. The clearance comes at a time of rising Coronavirus cases across the country, with 11.4 million cases recorded until Tuesday and more than 2,49,000 deaths.
"This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission." The FDA Commissioner Stephen Hahn said in a statement.
The product, authorized for use in hospitals and point-of-care settings, unlike antigen tests are highly reliable and according to Lucira Health, the tests have a sensitivity of about 94.1 per cent and a specificity of 98 per cent.