Roche's COVID antibody cocktail is now available in India
text_fieldsMumbai: Roche India on Monday announced the rollout of its first batch of the antibody cocktail (Casirivimab and Imdevimab) against Coronavirus in India.
The second batch of the cocktail jabs marketed by Cipla pan-India will be made available by mid-June.
The price for each patient dose [a combined dose of 1200 mg (600 mg of Casirivimab and 600 mg of Imdevimab)] will be Rs 59,750. The maximum retail price for the multi-dose pack (each pack can treat two patients) is Rs 119,500, said the statement released by the company.
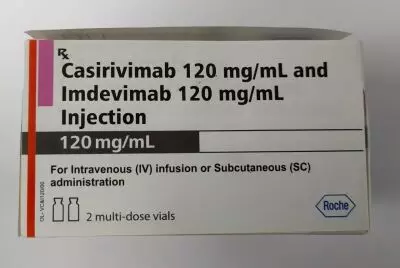
The Casirivimab-Imdevimab injection is a drug of two monoclonal antibodies which are produced by recombinant DNA technology and is designed specifically to block the infectivity of SARS-CoV-2, the virus which causes Covid-19. The monoclonal antibodies are produced by recombinant DNA technology.
The antibody cocktail drug can help in the treatment of mild to moderate coronavirus disease in adults and children aged 12 years or older and weighing at least 40 kg, those who are at high risk of developing severe Covid-19 disease, and do not require oxygen, the company said.
The jab is also noted to benefit high-risk patients before their condition worsens -- reducing the risk of hospitalisation and fatality by 70 per cent and shortening the duration of symptoms by four days.
"Roche is deeply committed to supporting the ongoing efforts to combat the Covid-19 pandemic, mitigate the deadly second wave and save lives. We are optimistic that the availability of antibody cocktail (Casirivimab and Imdevimab) in India can help in minimising hospitalisation, ease the burden on healthcare systems and play a key role in the treatment of high-risk patients before their condition worsens," said V Simpson Emmanuel, Managing Director and CEO, Roche Pharma India, in the statement.
Earlier this month, the Central Drugs Standards Control Organisation (CDSCO) had provided an Emergency Use Authorisation (EUA) for the antibody cocktail (Casirivimab and Imdevimab) in India. It has also received a EUA in the US and several EU countries.
Each pack of antibody cocktail (Casirivimab and Imdevimab) contains one vial of Casirivimab and one vial of Imdevimab totalling 2400 mg of the antibody cocktail (one vial of Casirivimab (1200 mg) and one vial of Imdevimab (1200 mg) and can treat two patients as the dosage per patient is a combined dose of 1200 mg (600 mg of Casirivimab and 600 mg of Imdevimab) administered by intravenous infusion or subcutaneous route.
The vials need to be stored at 2 degrees Celsius to 8 degrees Celsius. If opened for the first patients' dose, a vial can be used for the second patients' dose within 48 hours if stored at 2 degrees Celsius to 8 degrees Celsius, the company said.
(With IANS inputs)