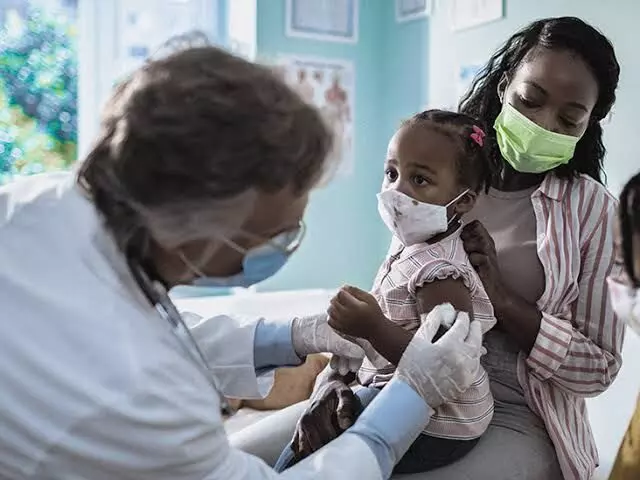
Benefits outweigh risks: FDA on Pfizer vaccine in children
text_fieldsThe US Food and Drugs Administration has voiced approval for administering the Pfizer-BioNTech vaccine to children in the 5-11 age group, citing data that shows the benefits of the vaccine outweigh the risks involved. On Friday, Pfizer had released data claiming 90.7% effectiveness of the vaccine against the Covid-19 virus in their clinical trial of 5-11 year olds.
The Pfizer vaccine has been linked to 'myocarditis' or heart inflammation in male patients. The company has submitted files and data as part of a scheduled meeting on October 26 with the FDA Vaccines and Related Biological Products Advisory Committee. The drop in hospitalisation and deaths in the children's trial was more than enough reason to administer the vaccine as it massively outweighed the risk of heart inflammation cases in children the FDA said.
The data show that the two-dose primary series of the vaccine given to children from 5 to less than 12 years of age confers a high degree of protective efficacy against Covid-19 during a period when the Delta variant of concern predominates in the US.
If approved, this could be the first vaccine for children of that age group. shots could be available in the United States in early November.